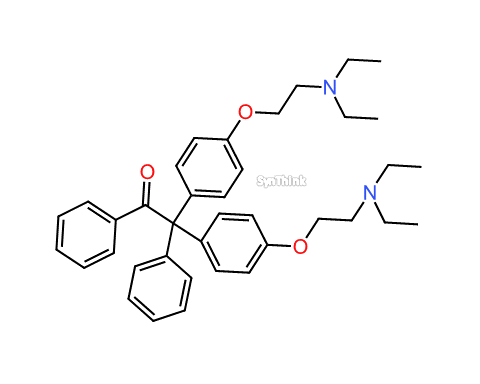
Clomifene EP Impurity D
| CAS No. | : 1391054-64-0 |
| Mol F. | : C38H46N2O3 |
| Mol W. | : 578.78 |
| Cat No. | : SA21704 |
- Description
- Quick Enquiry / RFQ
Description
| Product Name | Clomifene EP Impurity D |
| Synonyms | Clomiphene EP Impurity D
2,2-Bis[p-[2-(diethylamino)ethoxy]phenyl]-2-phenylacetophenone 2,2-Bis[4-[2-(diethylamino)ethoxy]phenyl]-1,2-diphenylethanone |
| Related API | Clomifene |
| Category | Impurities |
Clomifene EP Impurity D product with CAS: 1391054-64-0 is also known as Clomiphene EP Impurity D. This product can be used as a working standard or secondary reference standard. Additional internal validation as per respective FDA regulations/guidelines may require. Clomifene EP Impurity D is generally used for Quality Control (QC), Quality Assurance (QA) during commercial production of Clomifene and its related formulations. Moreover, Clomiphene EP Impurity D is also used in the process of Abbreviated New Drug Application (ANDA) filing to FDA and toxicity study of respective drug formulation.
If you need different pack size / quantity, please contact us or email us at enquiry@synthinkchemicals.com